What is an electrolyte and why use it?
This is an acid or alkali based liquid. It works as a current conductor. As the battery charge level increases, the density of the liquid increases.
The chemical solution must be of high quality, made without deviations from manufacturing technology. Otherwise, the battery will not charge.
Refilling electrolyte
The prepared solution should be poured into the battery through a funnel made of neutral material.
Fill the fluid one by one into the battery sections. We make the same level in all banks. The level should be from 1 to 1.5 cm above the plates. We do not touch the battery for 2-3 hours. The density may decrease slightly during standing.
Next, you should charge the car battery to operating parameters. To properly charge a charged battery, you must set the current to a value 10 times less than that indicated on the battery case. For example, if on the battery case it says 65 A*h (amps multiplied by an hour), then on the charger we set the current to 6.5 A (amps). At this value, it must be charged for 4 hours. After charging, we measure the density again.
What types of chemical liquid exist
Since an electrolyte is a mixture either based on an acid or an alkali, there are two types of this mixture: alkaline and acidic.
Acidic - a symbiosis of acid and distilled water. Batteries with this type of electrolyte are needed to start the engine.
The alkaline type of liquid uses a mixture of calcium-lithium base and distilled water.
Such mixtures serve to accumulate electricity in the battery. The scope of application of these batteries is electrical appliances, forklifts, and military vehicles.
Why does the battery fail?
Closing the topic of electrolytes, let us point out why even the most durable German battery can fail. Some of the reasons are related to refilling the battery, and some are related to violation of the simplest operating rules . Here's what car enthusiasts should know:
- It is strictly not recommended to dilute the electrolyte even with pure water - only with distillate. Filtered water contains small amounts of salts and metals that will seriously reduce battery life. The distillate can be bought either at a reputable auto chemical store or at a pharmacy;
- The battery with drained electrolyte was cleaned with a rag or sponge. It is permissible to use a soft cloth pre-moistened in ammonia or a solution of soda ash. In other cases, dirt will definitely remain inside;
- It is prohibited to use a solution of water and technical sulfuric acid as an electrolyte. It does not undergo the same fine cleaning as battery acid and is not suitable ;
- The voltage in the vehicle's on-board network should not be too high or too low. This seriously affects the battery life;
- It is strictly forbidden to use low-density electrolyte in winter or excessively add distillate to the old electrolyte. So the car owner can cause the destruction of the monoblock and the leakage of the solution immediately after thawing;
- Do not use an electrolyte that is too dense. If you do not use a balanced solution of acid and water, the battery will be destroyed from the inside;
- It is extremely dangerous to use too powerful starting devices. Too high a starting current instantly destroys the battery - the electrolyte in it boils with the formation of a large amount of gases that do not have time to escape from the ventilation holes. There are several known cases where batteries naturally exploded after using powerful launchers;
- In summer, it is better not to leave the car in the open sun. If the battery under the hood overheats, the electrodes in it will begin to wear out quickly. The battery life will begin to decline catastrophically quickly;
- The battery cannot be charged with high current. Under the influence of a large charging current, the electrolyte overheats . You can also observe the destruction of the separator, warping of the electrodes, and shedding of the active mass.
Method for preparing an acid solution
You will need the following:
- Mixing container and spatula designed to resist acid attack.
- Purified (distilled) water.
- Sulfuric acid for batteries.
- Protective clothing (apron, glasses, gloves).
- Baking soda (acid neutralizer).
How to prepare:
- Pour the required amount of water into the bowl and carefully and slowly pour the acid into the water.
- Mix with a spatula.
- Let it sit for at least half a day. For a liter of mixture we take 0.75 water and 0.285 acid.
Preparing an alkaline solution
We will need dishes that are resistant to chemical alkaline reactions, alkali (caustic potassium or caustic sodium), you can add lithium substances to improve the quality of the solution, water.
Important! We add completely purified water, i.e. distilled!
Cooking method:
- Pour distilled water into the bowl.
- Pour in the lye and stir thoroughly. If the density is unsatisfactory, then add alkali or liquid.
- We insist for three hours.
- Pour the resulting liquid into another vessel without raising the sediment.
Electrolyte with increased density
We may need it to correct the density of what is in the battery. If you have added a lot of water to the battery jars and the density of the chemical mixture has dropped, you can correct it using an electrolyte with a higher density.
To prepare, slightly change the proportions of alkali (acid) and water (0.423 parts per 0.650 water). The sequence of actions is the same as for making the main solution.
The chemical properties of the electrolyte are the same, but the freezing point is lower. The mixture is used only to adjust the main electrolyte.
Operating rules
The properties of the electrolyte are quite sensitive to changes in environmental temperature conditions, so in areas with a temperate climate it is recommended to check its condition twice a year: at the end of autumn and at the end of spring.
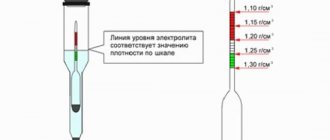
Density measurement
Density is an important characteristic of an acid electrolyte, the composition of which determines its value. The device that measures the density of the electrolyte is called a hydrometer, which can be bought at any auto store. When using it, the ambient temperature and the associated correction factor must be taken into account.
The following table shows the correction factors to the obtained hydrometer readings depending on the temperature (degrees Celsius):
- -40 to -26: -0.04;
- -25 to -11: -0.03;
- -10 to +4: -0.02;
- from +5 to +19: -0.01;
- +20 to +30: 0.00;
- from +31 to +45: +0.01.
In addition to the hydrometer, it is recommended to prepare a blank sheet of paper and a pencil in advance to record the measured results. The check must be carried out in each battery cell separately. The following steps explain the procedure:
- The first step is to open each container in the battery, the density of the electrolyte in which should be measured.
- The part of the hydrometer intended for measurement must be placed in an electrolyte.
- Using the instrument's bulb, you should pick up a certain portion of the electrolyte so that the hydrometer float begins to float.
- At the point of contact between the special rod and the liquid, you should look at the actual readings of the measured value.
- Record the result obtained, and then carry out similar actions for the remaining battery capacities.
We recommend: Ista battery: types of car batteries
Density is a physical quantity , the dimension of which is defined as g/cm3.
In the case of an electrolyte, after the measurements have been taken, you should make sure that its fluctuations in all battery elements do not exceed 0.2-0.3 g/cm3. If the average density value for all battery capacities is below the specified value in the passport, then it is necessary to charge the battery. When caring for the battery and monitoring the density of the electrolyte, it is necessary to keep in mind the temperature regime . Thus, in the cold season, higher values of this value (1.30 g/cm3) should be maintained, since it provides a lower freezing point of the liquid. For example, if the density value is below 1.1 g/cm3, then ice crystals may appear in the electrolyte already at a temperature of -6 °C. In summer, it is better to reduce the density of the charged battery to the level of 1.23 g/cm3, since the lower it is, the longer the device will last.
In winter, at low air temperatures, it is recommended to remove the battery from the car and bring it into a room in which all monitoring measurements of electrolytic parameters should be carried out. In addition, to operate an electrical device in the northern regions of the country, you should purchase a special jacket container, which allows you to retain the heat of the battery case.
Liquid level
Another key battery characteristic that needs to be monitored regularly is the electrolyte level in each cell.
According to general recommendations, it should not be lower than 1-1.5 cm of the upper edge of the plates. Before measuring the electrolyte level in each battery section, place the electrical device on a horizontal surface. After this, it is recommended to take a glass tube 25-30 cm long and 5-6 mm in diameter, lower it to the bottom of the jar being measured, close the free end of the tube with your thumb to prevent the liquid in it from falling when pulled out of the jar, and then pull it out of the electrolyte and Use any ruler to measure the level.
This operation can be carried out using a regular sheet of paper, which should be rolled into a tube and lowered to the bottom of the container being measured. When subsequently measuring a wet print on a sheet with a ruler, the amount of error arising due to the capillary effect should be taken into account.
If during measurements a lack of liquid is detected in any battery capacity, then the required amount of distilled water should be added to it.
This should be done carefully, in small portions, since water entering the acid causes a large release of heat and boiling. It is necessary to add water, not electrolyte, otherwise the electrical appliance may be seriously damaged.
Which batteries use different types of chemical fluid?
To avoid mistakes, you should study the label on the battery. If the battery is lead-acid type, then we use an acid mixture.
We pour a solution based on potassium or sodium into alkaline batteries. The type of alkali can be recognized by its combustion. Potassium burns violet-red and sodium burns yellow.
So, if suddenly the need arises to prepare the electrolyte for the battery yourself, then, in principle, this is possible. You should follow safety precautions and be attentive to the proportions of substances - and everything will definitely work out.