What is an electrolyte and why use it?
This is an acid or alkali based liquid. It works as a current conductor. As the battery charge level increases, the density of the liquid increases.
The chemical solution must be of high quality, made without deviations from manufacturing technology. Otherwise, the battery will not charge.
Chemical reactions taking place
Lead-acid batteries are widely used due to their simple design. The accumulation of charge and its release occurs due to electrochemical processes that can only occur in the presence of an electrolyte. The features of such power sources include the following:
- The design is represented by a combination of positive and negative plates, which are immersed in an aqueous solution of hydrochloric acid.
- The plates have current-conducting grids. In their manufacture, lead is used, to which various alloying elements are added. The type of additives used depends on the type of battery.
- The positive electrode grid is coated with lead dioxide, the negative plates are coated with black powder.
- In many ways, the electrical characteristics of batteries depend on the density of the electrolyte used.
To protect the structure from external factors, a sealed housing is created, which has an opening for gases to escape. The electrolyte can change its density depending on the state of charge of the battery.
The machines use a battery that uses acid with a density of 1.835 g/cm3. When distilled water is added, the density becomes 1.3 g/cm3. Severe discharge of the power source leads to a drop in density due to a decrease in the amount of acid.
We recommend: Ways to check a car battery at home
What types of chemical liquid exist
Since an electrolyte is a mixture either based on an acid or an alkali, there are two types of this mixture: alkaline and acidic.
Acidic - a symbiosis of acid and distilled water. Batteries with this type of electrolyte are needed to start the engine.
The alkaline type of liquid uses a mixture of calcium-lithium base and distilled water.
Such mixtures serve to accumulate electricity in the battery. The scope of application of these batteries is electrical appliances, forklifts, and military vehicles.
How to monitor the condition of the battery
The motorist faces a very important task. He needs to constantly monitor the condition of the battery, monitor the level of working fluid, its density and concentration.
All this can be controlled and influenced when using a lead-acid serviced battery.
If the battery is maintenance-free, adding water or filling in the missing amount of electrolyte will not work. Some use tricks, make small holes, and use medical syringes. But this is potentially very dangerous and it is strongly not recommended to engage in such restoration of maintenance-free batteries.
When the battery is serviced, monitoring the level and density is not difficult. To check the level you need:
- unscrew the caps on the cans;
- take a tube made of glass or transparent plastic;
- immerse the tool in the battery up to the plates;
- pinch the top hole of the tube with your finger;
- lift the device;
- measure at what level the working fluid is.
The norm is considered to be a value ranging from 10 to 15 mm. It is important to note that many batteries have special marks that make it quite easy to check the level.
If the level differs from normal, then you will need to perform the procedure of adding electrolyte or only distilled water. This depends on what the density measurements show.
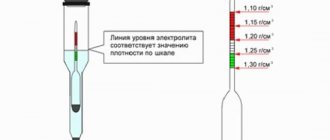
When measuring density, you cannot do without such a device as a hydrometer. It is immersed in all battery banks, and the parameters in each of them are checked. If the density is higher than the recommended values, the composition should be diluted with distilled water. If the density is below normal, then electrolyte is added.
Method for preparing an acid solution
You will need the following:
- Mixing container and spatula designed to resist acid attack.
- Purified (distilled) water.
- Sulfuric acid for batteries.
- Protective clothing (apron, glasses, gloves).
- Baking soda (acid neutralizer).
How to prepare:
- Pour the required amount of water into the bowl and carefully and slowly pour the acid into the water.
- Mix with a spatula.
- Let it sit for at least half a day. For a liter of mixture we take 0.75 water and 0.285 acid.
Why is this acid used in batteries?
Sulfuric acid is the most active inorganic acid, which reacts with almost all metals and their oxides. Without this, it will be completely impossible to discharge and charge the battery. However, how the charging and discharging processes will occur depends on the amount of distilled water with which the acid is diluted.
Or... The summary we can give on the question of what kind of acid is in batteries is as follows:
Every lead-acid battery contains sulfuric acid. This (acid) is not in pure form, but in diluted form and is called an electrolyte.