Six naive questions about phosphorus ammunition
1. What are phosphorus ammunition?
This is a type of incendiary or smoke ammunition filled with white phosphorus.
2. What is white phosphorus?
White phosphorus is a solid, waxy, poisonous substance that ignites spontaneously in air. Capable of self-ignition when combining with oxygen in the air. It burns (temperature 800-900°C), emitting thick and acrid white smoke, causing burns and poisoning of the body. It is used to equip incendiary and smoke shells, mines, aerial bombs, as well as to ignite and enhance the effect of napalm incendiary mixtures. White phosphorus and its vapors have toxic properties; a dose of 0.1 g causes death. In appearance, it is very similar to refined wax or paraffin, is easy to cut with a knife and is deformed with little effort. If it comes into contact with a person, it causes damage to bones, bone marrow, necrosis of the jaws).
3. In what form do phosphorus ammunition exist?
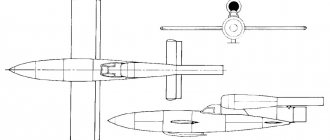
There are several types of such ammunition: aerial bombs, artillery shells, rockets (missiles), mortar shells, hand grenades.
4. How do phosphorus munitions affect people?
A projectile containing white phosphorus, after an explosion at a certain height, scatters the ignited substance over a large area, the area of which can reach 150-200 square meters. The combustion temperature of the substance exceeds 800 degrees Celsius. Combustion continues until all phosphorus is burned out or until oxygen supply ceases. Such weapons can cause particularly severe and painful injuries or provoke a slow and painful death. Treatment of such injuries requires specially trained medical personnel, who may also receive phosphorus wounds while working. The use of white phosphorus gives a “complex effect” - not only smoke and flame, but also psychological shock. A characteristic feature of the use of a phosphorus bomb is the charring of human skin and bones, and when inhaling the burning mixture, it burns out the lungs. An incendiary mine fired from a conventional mortar, upon explosion, showers the target with a sheaf of sparks, ash, burning incendiary equipment (phosphorus), flame, rain of molten metal or slag (thermite). Mines can also be filled with mixtures of incendiary substances, for example, pieces of coal tar mixed with phosphorus, TNT, dissolved in carbon disulfide, a self-igniting substance. Such mines burn very intensely for several minutes, producing strong smoke.
Viktor Baranets, a military observer for the Communist Party, explained how the Ukrainian army could have this kind of weapon
00:00
00:00
5.Are phosphorus ammunition a prohibited weapon?
When used against military targets located “in or in the vicinity of populated areas,” weapons containing white phosphorus are prohibited for use under international agreements (Protocol III to the 2006 Geneva Convention on Certain Conventional Weapons). Smoke munitions are not covered by the Convention.
The traditional evening shelling of the suburbs of Slavyansk began last night around 23:00. First the mortars started talking, followed by the tanks, then the heavy artillery. And around midnight, the sky suddenly lit up with many luminous balls, which, scattering in bunches, Photo: Alexander KOTS
6. When and where were phosphorus ammunition used?
The combat use of such ammunition has been known since the 19th century. White phosphorus was used by Irish independence fighters in the fight against the British regime. During World War I, all sides used white phosphorus incendiary bullets, primarily for shooting at aerial targets. In 1916, incendiary grenades filled with white phosphorus entered service with British troops.
During World War II, white phosphorus was widely used to fuel incendiary bombs. In 1982, 155-mm artillery shells filled with white phosphorus were used by the Israeli army during the Lebanon War (in particular, during the siege of Beirut). The Nicaraguan Contras repeatedly used American-made mines and hand grenades filled with white phosphorus: for example, in April 1984, in the area of the Bluefields port, two Contra saboteurs were blown up while trying to plant mines filled with white phosphorus. In early June 1985, about 70 contras stopped and captured the Bluefields Express passenger ship sailing along the Escondido River. Since five SNA servicemen who provided security for the voyage barricaded themselves in the hold and engine room and did not allow the ship to be taken away along the river, the contras burned the ship with American phosphorus grenades (losses from the loss of the ship amounted to 500 thousand US dollars). In September 1985, the Contras burned down a grain warehouse-elevator in the village of Palacaguina with white phosphorus grenades, containing 130 tons of rice, beans and corn. The Vietnamese People's Army used white phosphorus against Khmer Rouge guerrillas during the occupation of Kampuchea in the 1980s. They were equipped with unguided missiles, which Vietnamese aircraft used to attack ground targets, and grenades, used from light aircraft in a similar way. During the siege of Sarajevo, phosphorus shells were used by Bosnian Serb artillery. In 1992, the building of the Institute of Oriental Studies was burned with such shells, resulting in the destruction of many historical documents. In 2004, the US used it against militants in Iraq in the battle for Fallujah. In the summer of 2006, during the Second Lebanon War, artillery shells containing white phosphorus were used by the Israeli army. Lebanese President Emile Lahoud issued a statement that civilians were injured as a result of the Israelis using phosphorus shells. After this, a representative of the Israeli government issued a statement that phosphorus shells were used “only on military targets.” In 2009, during Operation Cast Lead in the Gaza Strip, the Israeli army used smoke munitions containing white phosphorus. In 2009, Palestinian rebels charged their missiles with white phosphorus. The use of phosphorus ammunition by the Ukrainian army near Slavyansk is the first case in the world in the last 5 years.
x HTML code
Slavyansk militia: Ukraine used phosphorus ammunition. Archive footage from June 2014, it was this footage (sources without editing) that the Investigative Committee of the Russian Federation attached to the case of military crimes in Ukraine Alexander KOTS, Dmitry STESHIN
White phosphorus
Among all the flammable substances used in incendiary ammunition, white phosphorus occupies a special place. This is due to its unique chemical properties and, first of all, its combustion temperature, reaching 800-1000 degrees Celsius. Another important factor is the ability of this substance to spontaneously ignite when interacting with oxygen in the air. When burned, white phosphorus emits thick, toxic smoke, which also causes burns to the internal respiratory tract and poisoning of the body.
A dose of 0.05-0.1 g is lethal for humans. White phosphorus is obtained artificially by reacting phosphorites or apatites with silica and coke at a temperature of 1600 degrees. Outwardly, it looks like paraffin, is easily deformed and cut, which makes it very convenient for equipping any ammunition. There are also bombs filled with plasticized white phosphorus. Plasticization is achieved by adding a viscous solution
story
A member of the US Air Force inspects a 2.75-inch white phosphorus missile marking at Osan Air Base, South Korea in 1996
White phosphorus is believed to have been first used by Fenian arsonists in the 19th century, in the form of a solution in carbon disulfide. When the carbon disulfide was evaporated, the phosphorus would ignite. This mixture was known as "Fenian fire".
In 1916, during the intense struggle for conscription for the First World War, 12 members of the Industrial Workers of the World, a union of workers distinguished from conscription, were arrested in Sydney, Australia and convicted of using or conspiring to use incendiary materials, including including phosphorus. It is believed that eight or nine people from this group, known as the Sydney Twelve, were rounded up by police. Most of them were released in 1920 after request.
Known facts about the use of phosphorus ammunition in our time
During the occupation of Kampuchea in the 1980s of the last century, the Vietnamese army used aircraft unguided rockets charged with white phosphorus to destroy the Khmer Rouge. Phosphorus rockets were used by British intelligence agencies in 2003 near the city of Basra in Iraq.
A year later, in Iraq, the US Army used phosphorus bombs in the battles for Fallujah. You can see photos of the consequences of this bombing in the article. In 2006 and 2009, the Israeli army used phosphorus munitions during the Second Lebanon War, as well as in the Gaza Strip during Operation Cast Lead.
Dangerous first aid
An unprepared person cannot provide assistance to a victim of white phosphorus; rather, he himself will receive burns from this toxic substance. Anyone who inhales white phosphorus vapor is practically doomed - damage to the upper respiratory tract occurs. In this case, a severe spasm often occurs, which leads to death.
An American Douglas A-1 Skyraider attack aircraft strikes Viet Cong positions with phosphorus ammunition. Photo: wikipedia.org
The use of shells containing white phosphorus leads to large casualties among civilians. After the ammunition ruptures, the fragments penetrate deeply into the body and it is often impossible to save the person.
Those affected by white phosphorus are characterized by “face-hands” syndrome, when a person tries to remove the burning mixture from the face with bare hands. As a result, the victim receives severe burns to the hands, which are accompanied by severe pain.
Damaging factors
When using white phosphorus as a flammable substance for an aerial bomb, several damaging factors are obtained:
- strong flame from burning the mixture at temperatures up to 2000 ˚ C, causing burns, terrible injuries and painful death;
- stimulating spasms and burning of the airways;
- oxygen burnout in the area of use, leading to suffocation;
- psychological shock caused by what he saw.
A small phosphorus bomb, detonated at the right height, hits an area of 100-200 square meters, covering everything around with fire. Once on the human body, particles of burning slag and phosphorus stick and char organic tissue. You can stop the combustion by cutting off the access of oxygen.
Special phosphorus landmines are also used to defeat an enemy in cover. A flammable mixture heated to 1500-2000 C can burn through armor and even concrete floors, and given that at this temperature the oxygen in the air quickly burns out, there is practically no chance of surviving by hiding in a basement, dugout or other shelter.
It was from suffocation that hundreds of Vietnamese civilians died during one of the US Air Force bombings. These people found their death in pre-dug dugouts, having no idea what a phosphorus bomb was.
How to deal with the consequences
Before taking actions aimed at saving a person, it is necessary to make sure that phosphorus bombs and the substances they contain are really the cause. Such burns have a specific smell of garlic, the skin around them smokes and chars.
First of all, an aseptic bandage is applied to the extinguished burn to prevent inflammation and infection. Next, all measures are taken to prevent painful shock with the subsequent evacuation of the person from the affected area. During the cold season, it is not recommended to remove the victim’s clothing so as not to increase the shock.
The use of any medications without a preliminary analysis of a person’s condition is permissible only if the doctor knows for sure that the medicine is more likely to help than harm. However, experts strongly recommend not to provide assistance to the victim if the person does not know what to do with such injuries.
Video: phosphorus bomb
Used for combat operations, ammunition was required that could destroy enemy ground forces over a large area. Incendiary bombs appeared on the eve of the First World War. These were primitive devices consisting of a container with kerosene and an inertial fuse, the basis for which was an ordinary rifle cartridge.
In the 1930s, so-called phosphorus balloons were used for bombing. They were filled with yellow phosphorus in the form of granules measuring 15-20 mm. When such a ball was dropped, it was set on fire, and closer to the ground, the burning particles of phosphorus, having scorched the shell, scattered, covering a huge area with fiery rain. The method of spraying ignited granules from special aircraft tanks at low altitude was also used.
During World War II, humanity first learned what a phosphorus bomb is in the form in which it exists today. It was a container filled with phosphorus balls weighing from 100 to 300 g, with a total weight of up to one ton. Such ammunition was dropped from a height of about 2 km and exploded 300 m from the ground. Nowadays, incendiary shells based on phosphorus in the strongest armies of the world occupy a significant part of the total ammunition used for bombing.